A person may join in the Zostavax litigation if he meets three conditions. These are:
- he took the Zostavax drug after 2006 (the year it was licensed by the Food and Drug Administration (“FDA”)),
- he experienced shingles anywhere between three weeks to a year after taking the vaccine, and
- he was diagnosed with shingles by a licensed doctor or physician.
A person may also be able to join the Zostavax lawsuits if he suffered from a certain injury within two years from taking the medication. Some of these injuries and complications are:
- neurological disorders,
- blindness, and
- hearing loss.
The “Zostavax lawsuits” refer to the thousands of cases people have filed against the drug’s manufacturer since 2016. These suits mainly allege that:
- the drug is defectively designed, and
- the drug’s maker, Merck & Co Inc. Pharmaceuticals, failed to adequately warn about serious side effects.
A federal judicial panel recently consolidated many of these lawsuits in federal court and created the Zostavax lawsuit multi district litigation (“Zostavax MDL”).
Note that a plaintiff can get a new lawsuit transferred into the Zostavax MDL if:
- the suit is filed in federal civil court, and
- the facts of the case are similar to the facts of the other cases in the MDL.
All Zostavax lawsuits have an applicable statute of limitations (“SOL”). But not all lawsuits will have the same SOL. The precise SOL applicable to these suits will depend on:
- the state in which the suit is filed, and
- the legal theory upon which the lawsuit is based.
Zostavax is a vaccine designed to prevent shingles. The drug reduces the risk that people will develop this illness by 51percent – for appropriate patients aged 60 years or over. Efficacy of the medicine goes down as the age of a person goes up. It is administered via an injection into a patient’s upper arm.
Please note that two vaccines are licensed and recommended to prevent shingles in the United States – Zostavax and Shingrix. Shingrix is now recommended as the preferred shingles vaccine.
Our national mass tort lawsuit attorneys will highlight the following in this article:
- 1. Who qualifies to be a plaintiff in the Zostavax litigation?
- 2. What is the Zostavax litigation?
- 3. Can a new claim get transferred into the Zostavax MDL?
- 4. Should a new Zostavax case be filed in federal court or state court?
- 5. Is there a statute of limitations applicable to a Zostavax lawsuit?
- 6. What is Zostavax?
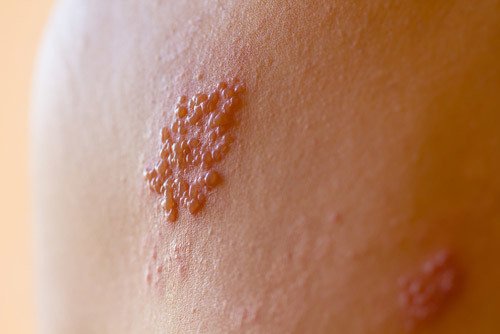
1. Who qualifies to be a plaintiff in the Zostavax litigation?
A person may join in the Zostavax litigation if he meets three conditions. These are:
- he took Zostavax after 2006 (the year it was licensed by the FDA),
- he experienced shingles anywhere between three weeks to a year after taking the vaccine, and
- he was diagnosed with shingles by a licensed doctor or physician.
A person may also be able to join the Zostavax litigation if he suffered from a certain injury within two years from taking the drug. Some of these injuries are:
- postherpetic neuralgia (“PHN”)
- stroke,
- hearing loss,
- congestive heart failure,
- blindness,
- certain neurological disorders,
- pneumonia, and
- death.
2. What is the Zostavax litigation?
Zostavax lawsuits are legal claims asserted by consumers of the drug against its manufacturer, Merck.
These suits mainly allege that the drug is defectively designed, and Merck failed to adequately warn about serious side effects. Plaintiffs are seeking compensation for injuries such as:
- development of shingles (the virus Zostavax was meant to prevent),
- hearing loss,
- blindness,
- paralysis, and
- fatal liver failure.
The first Zostavax lawsuit was filed in 2016.1 Since that time, thousands of similar suits have been filed across the nation.
A federal judicial panel recently consolidated many of the lawsuits in federal court and created the Zostavax lawsuit multi district litigation (“Zostavax MDL”).2 This litigation is before U.S. District Judge Harvey Bartle of the Eastern District of Pennsylvania. Judge Bartle has been overseeing the very first Zostavax case filed.3
In short, the plaintiffs in the Zostavax MDL state that:
- Zostavax, as designed, is unreasonably dangerous,
- the vaccine can cause serious injuries, including death,
- Merck failed to warn the public of the potential negative effects of using the drug, and
- the defendants willfully misrepresented the safety of the vaccine.
The order creating the Zostavax MDL does not apply to lawsuits brought on behalf of 300 patients in California state court and 800 plaintiffs in New Jersey state court.4
The first Zostavax trials are expected to take place sometime between fall 2020 and summer 2021.

3. Can a new claim get transferred into the Zostavax MDL?
A new lawsuit can get transferred into the Zostavax MDL if:
- the suit is filed in federal civil court, and
- the facts of the case are similar to the facts of the other cases in the MDL.
Civil cases are contrasted with criminal cases. Civil cases are those filed by an injured party, in civil court (and not criminal court), against the person or company who hurt him. Here, that party would be Merck.
In addition, federal cases are contrasted with state court cases. While federal cases are filed in federal court, state cases are filed in a state court.
Please also note that there must be a similarity between the facts of a new case and those existing cases within the MDL in order for a case to be transferred into it. There are no exceptions to this rule.
4. Should a new Zostavax case be filed in federal court or state court?
Any new Zostavax case can be filed in either federal or state court.
Please note, though, as mentioned above, a case must be filed within federal court if a plaintiff wants to transfer it into the Zostavax multidistrict litigation.
A case can be filed in federal court, rather than in state court, if either:
- the plaintiff and the defendant are from different states and the damages at issue total more than $75,000,5 or
- the case implicates federal law.6
5. Is there a statute of limitations applicable to a Zostavax lawsuit?
All Zostavax lawsuits do not have the same SOL. Note that the precise limitations period applicable to any Zostavax lawsuit will depend on:
- the state in which the suit is filed in, and
- the legal theory upon which the lawsuit is based.
Despite differing SOLs, though, please note that all Zostavax suits must be filed before their precise limitation period expires.
5.1. State the case is filed in
States do not always have the same SOL for the same cause of action, or reason supporting a lawsuit.
Since Zostavax lawsuits have been, and are, getting filed in multiple states across the nation, this means that one blanket statute of limitations cannot be given for all Zostavax lawsuits. The statute of limitations may vary depending on the laws of the particular state where the plaintiff decides to file the suit.
5.2. Legal theory forming the basis of the suit
A SOL may differ for legal theories forming the basis of a lawsuit.
Since Zostavax lawsuits are based upon differing legal theories, this means that one blanket statute of limitations cannot be given for all Zostavax lawsuits. The statute of limitations may vary depending on the particular cause of action a plaintiff is using.
A few legal theories being used in the Zostavax suits are:
- design defect,
- failure to warn,
- fraudulent misrepresentation,
- negligent, and
- unjust enrichment.
6. What is Zostavax?
Zostavax (or, zoster vaccine live) is a vaccine designed to prevent patients from contracting shingles. The drug is appropriate for patients aged 60 years or older. As stated above, the drug is manufactured by Merck & Co Inc. Pharmaceuticals.7
The Zostavax vaccine was licensed by the FDA in 2006. It is given to patients in a single dose and is administered as a shot. The medicine can be given in a doctor’s office or pharmacy.8
The vaccine, though, is only successful in about 51 percent of patients that take it. And, efficacy of the medicine goes down as the age of a person goes up.
Please note that two vaccines are licensed and recommended to prevent shingles in the United States – Zostavax and Shingrix. Shingrix is a newer drug that started to be administered in 2017. It is now recommended as the preferred shingles vaccine.
Did you experience an injury or adverse condition after taking Zostavax? Call us for help…

If you or someone you know has experienced an injury or adverse effect upon taking the Zostavax vaccine, we invite you to contact us for a free consultation. We can be reached 24/7.
Legal References:
- The Legal Intelligencer, “MDL Panel Sends Shingles Vaccine Lawsuits to Pennsylvania,” August 2, 2018.
- See same.
- See same.
- See same.
- 28 U.S.C. § 1332.
- 28 U.S.C. § 1331.
- Centers for Disease Control and Prevention (CDC), “What Everyone Should Know about Zostavax.” CDC website.
- See same.
