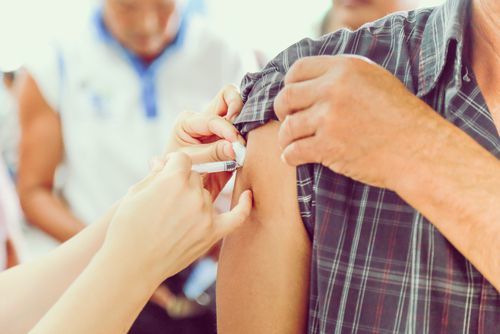
Zostavax is administered via an injection or shot into a patient’s upper arm. The drug is given to patients in a single dose. The dose is 0.65 mL, or one entire vial.
The vaccine is used for adults 50 years of age or older to prevent shingles. While licensed for patients 50 years and older, the Advisory Committee on Immunization Practices (“ACIP”) recommends that vaccination with Zostavax begin at 60 years of age.
The medicine is not recommended for people under the age of 50. In addition, the drug is not recommended for the following two categories of people:
- persons who have experienced a life-threatening reaction to gelatin, neomycin, or any other component of shingles vaccine, and
- persons with a weakened immune system due to certain conditions.
People taking the Zostavax medication have reported experiencing some of the following complications and side effects:
- shingles (or, the virus that Zostavax was meant to prevent),
- rash,
- hives,
- fever,
- nausea,
- headache, and
- joint and muscle pain.
In addition, thousands of Zostavax lawsuits have been filed against the drug’s manufacturer Merck & Co Inc. Pharmaceuticals since 2016. The plaintiffs in these lawsuits allege that, in addition to the above complications, the medication causes:
- neurological diseases or disorders, like brain inflammation (or encephalitis) and brain damage,
- spinal cord inflammation (or myelitis),
- hearing loss,
- blindness,
- liver damage and liver failure, and
- neuropathy.
Shingrix is another vaccine that is aimed to prevent a person from experiencing shingles. Today, the drug is the preferred medication to help patients aged 50 and older avoid shingle complications. It is recommended that patients get two doses of the drug, separated by 2 to 6 months
Even though Shingrix is the preferred vaccine, Zostavax is still being administered to patients.
Our national mass tort lawsuit attorneys will highlight the following in this article:
- 1. How is Zostavax administered?
- 2. Who is the medicine administered to?
- 3. Who should not get Zostavax?
- 4. Are there complications associated with the drug?
- 5. Are there other vaccines, besides Zostavax, that help prevent shingles?
- 6. Is Zostavax still being administered to patients?
1. How is Zostavax administered?
Zostavax is administered via an injection or shot into a patient’s upper arm. The drug is given to patients in a single dose. The dose is 0.65 mL, or one entire vial.
The medicine can be given in a doctor’s office or pharmacy.1
2. Who is the medicine administered to?
Zostavax is a vaccine that is used for adults 50 years of age or older to prevent shingles. Although licensed for patients 50 years and older, the ACIP recommends that vaccination with Zostavax begin at 60 years of age.2 One of the main reasons is that the medicine protects against shingles for about 5 years, so adults vaccinated before they are 60 might not be protected later in life when the risk for shingles is greatest.3
Please note that two vaccines are licensed and recommended to prevent shingles in the United States – Zostavax and Shingrix. While Zostavax has been used since 2006, Shingrix is a newer drug that started to be administered in 2017. Shingrix is now recommended by ACIP as the preferred shingles vaccine.
3. Who should not get Zostavax?
Zostavax was designed for patients aged 50 and over. Therefore, it is recommended that persons under the age of 50 should not take the medication.
In addition, according to the Centers for Disease Control and Prevention (CDC), the following two categories of persons should not take the drug:
- people who have ever experienced a life-threatening or severe allergic reaction to gelatin, the antibiotic neomycin, or any other component of shingles vaccine, and
- persons who have a weakened immune system because of:
- HIV/Aids or another disease that affects the immune system,
- treatment with drugs that affect the immune system (e.g., steroids),
- cancer treatment such as radiation or chemotherapy, or
- cancer affecting the bone marrow or lymphatic system, such as leukemia or lymphoma.4
Please also note that women should not become pregnant until at least four weeks after getting Zostavax.5
In addition, people with a minor illness, like the flu, may be vaccinated with the drug. But, people with a moderate to severe illness should wait before taking Zostavax until they recover from their sickness. 6
4. Are there complications associated with the drug?
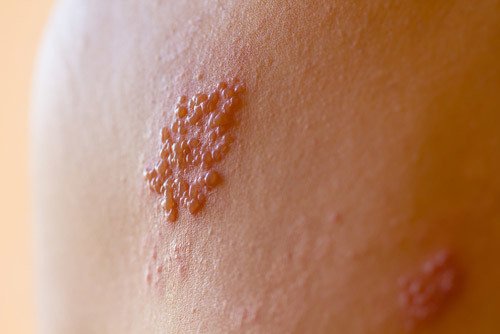
People taking the Zostavax medication have reported experiencing some of the following complications and side effects:
- shingles (or, the virus that Zostavax was meant to prevent),
- rash,
- hives,
- fever,
- nausea,
- headache, and
- joint and muscle pain.7
In addition, thousands of Zostavax lawsuits have been filed against the drug’s manufacturer Merck & Co Inc. Pharmaceuticals since 2016. The plaintiffs in these lawsuits allege that, in addition to the above complications, the medication causes:
- neurological diseases or disorders, like brain inflammation (or encephalitis) and brain damage,
- spinal cord inflammation (or myelitis),
- hearing loss,
- blindness,
- liver damage and liver failure, and
- neuropathy.
Zostavax may also cause immunocompromised patients to develop shingles.
5. Are there other vaccines, besides Zostavax, that help prevent shingles?
As mentioned above, Shingrix is another vaccine that is aimed to prevent a person from experiencing shingles. The vaccine uses a recombinant design as opposed to a live design.
The CDC states that Shingrix is the preferred drug over Zostavax. CDC recommends that healthy adults 50 years and older should get two doses of Shingrix, separated by 2 to 6 months, to best fight off the Shingles illness.8
According to CDC:
- two doses of Shingrix is more than 90 percent effective at preventing shingles, and
- protection stays above 85 percent for at least the first four years after a patient gets vaccinated.9
In one trial that involved more than 15,000 patients (aged 50 or older), Shingrix’s efficacy for preventing Shingles was greater than 95 percent.10 The efficacy of Zostavax is only about 51 percent. In a companion trial that included almost 14,000 patients (aged 70 or older), efficacy was about 90 percent.11
6. Is Zostavax still being administered to patients?
Shingrix is now the “preferred” drug over Zostavax. This does not mean, however, that Zostavax is no longer used to help prevent shingles. The medicine may still be given to adults aged 60 years old or over in certain cases.12
Examples of when Zostavax may still be used include:
- when Shingrix is not available,
- a patient prefers it over other medicines, and/or
- a patient is allergic to Shingrix.13
Did you experience an injury or adverse condition after taking Zostavax? Call us for help…

If you or someone you know has experienced an injury or adverse effect upon taking the Zostavax vaccine, we invite you to contact us for a free consultation. We can be reached 24/7.
Legal References:
- Centers for Disease Control and Prevention (CDC), “What Everyone Should Know about Zostavax.” CDC website.
- “Zoster Vaccine Live (Rx),” Reference.Medscape.com.
- Centers for Disease Control and Prevention (CDC), “What Everyone Should Know about Zostavax.” CDC website.
- Centers for Disease Control and Prevention (CDC), “What Everyone Should Know about Zostavax.” CDC website.
- See same.
- See same.
- U.S. Food and Drug Administration, “Zostavax.” FDA website.
- Centers for Disease Control and Prevention (CDC), “What Everyone Should Know about Shingrix.” CDC website.
- See same.
- Abigail Zuger, MD, “New Shingles Vaccine is Here!” New England Journal of Medicine.
- See same.
- “Zoster (Shingles),” Immunize.org.
- See same.
