Suboxone (buprenorphine and naloxone) is meant to treat opioid use disorder, but it causes patients serious teeth and gum injuries that require painful and expensive dental work.
Shouse Law Group is suing Indivior, the maker of Suboxone, in pursuit of the largest financial settlement possible for Suboxone victims. We are accepting clients across the United States who:
- have been prescribed Suboxone (as a sublingual film) for opioid addiction or pain management; and
- used prescription Suboxone for at least six months before suffering injuries; and
- have one or more of the following injuries: Cavities, tooth loss, tooth fractures, tooth decay, oral surgeries, tongue injuries and gum injuries; and
- have had routine dental care prior to Suboxone usage; and
- have no reported use of methamphetamine and/or convictions related to methamphetamine prior to diagnosis.
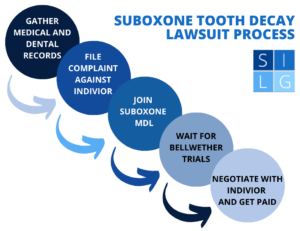
In this article, our Suboxone attorneys answer these frequently asked questions:
- 1. Does Suboxone cause dental problems?
- 2. Has Suboxone been recalled?
- 3. What are my legal grounds?
- 4. What is the current state of Suboxone litigation?
- 5. How much money can I get if I sue?
- 6. What are the side effects of Suboxone?
- 7. Who makes Suboxone?
- 8. What if I need Suboxone?
- 9. How does Suboxone work?
- 10. How can an attorney help me?
1. Does Suboxone cause dental problems?
There is strong evidence that Suboxone causes:
- oral infections,
- tooth decay,
- dental caries (such as loss of enamel or dentine),
- cavities,
- tooth fractures,
- tooth loss, and
- other tooth or gum injuries.
It takes several minutes for the Suboxone film to dissolve under your tongue, which is enough time to wreak havoc on your teeth and gums.
Suboxone lessens your salivary flow and your mouth’s buffering capacity. Suboxone is also extremely acidic (with a 3.4 pH in water), alters your oral pH, and causes immunosuppressive effects.
According to one 2022 study, people who used sublingual buprenorphine (like Suboxone) were at a:
- 1.42 times higher risk of dental problems as compared to those using transdermal buprenorphine, and
- 1.67 times higher risk of dental problems as compared to those using oral naltrexone.1
Potential dental work
Ultimately, many Suboxone patients develop severe and permanent teeth and gum problems that require extensive and expensive dental, periodontal, endodontal, and/or reconstructive/cosmetic work such as:
- fillings,
- crowns or crown replacements,
- root canals,
- tooth extractions,
- veneers,
- implants, and/or
- other oral surgeries.

Evidence is mounting that Suboxone causes dental damage.
2. Has Suboxone been recalled?
No, Suboxone remains available to purchase with a doctor’s prescription. However, on January 12, 2022, the U.S. Food and Drug Administration (FDA) issued a warning that adverse dental effects may be caused by medications containing buprenorphine such as Suboxone.
The FDA specified that they were aware of more than 305 cases of tooth decay/loss and oral infections related to buprenorphine use, and more than 131 of those cases were serious. The FDA also ordered that pharmaceutical companies update the prescribing information of all buprenorphine medications to include adverse dental effects.
However, the official Suboxone website still hardly mentions dental issues:
- All the main page says is for patients to inform their healthcare provider if they have a history of “tooth problems, including a history of cavities.”
- All the medication guide says is, “Report any problems with your teeth immediately to your healthcare provider and schedule an appointment with a dentist. Tell your dentist that you have started taking SUBOXONE.”
3. What are my legal grounds?
The Suboxone lawsuits are based on four causes of action:
- Strict liability failure to warn,
- Negligent failure to warn,
- Strict liability design defect, and
- Negligent design defect.
The strongest of these claims is that before 2022, Indivior failed to warn doctors and patients about Suboxone’s dental risks. Had patients known of the risks, they might have chosen to avoid the drug altogether.
Indivior should have known there was a problem as early as 2010 when they submitted to the FDA close to 20 reports of Suboxone users who developed dental problems. Some case reports were published in 2012, identifying 11 Suboxone patients whose dental health markedly declined.2
4. What is the current state of Suboxone litigation?
Suboxone victims have started filing individual lawsuits in September of 2023 against Indivior, Inc. and the following co-defendants:
- Indivior PLC
- Indiviorsolutions, Inc.
- Reckitt Benckiser, LLC
- Reckittbenckiser Healthcare (UL), LTD
- Aquestive Therapeutics, Inc.
- MonoSol, LLC
- MonoSol Rx, Inc. (which changed its name to Aquestive Therapeutics, Inc.)3
As more victims file Suboxone lawsuits, there is a good chance that all these lawsuits will be joined in a multi-district litigation (MDL) to help expedite the litigation process. Usually four or five representative cases go to trial, and how these “bellwether trials” turn out serve as the starting point for negotiations with the remaining plaintiffs.
5. How much money can I get if I sue?
If we bring a Suboxone lawsuit on your behalf, we will fight for all of the following compensatory damages:
- Medical bills for all the dental work you have had done and may need in the future due to the Suboxone;
- Lost wages from the time you had to take off work due to your dental issues;
- Loss of future earnings for work you anticipate not being able to do because of your dental issues;
- Pain and suffering from the emotional distress of having dental problems; and
- Any other out-of-pocket expenses you incurred due to the Suboxone.
If you relapsed to using opioids, we will also seek reimbursements for all the costs associated with your rehablitation. In a cruel twist of irony, Suboxone victims’ painful dental problems may drive them to self-medicate with the very opioid painkillers that Suboxone was supposed to help them break free of.
Punitive damages for Suboxone lawsuits
In addition to compensatory damages, we seek extensive punitive damages to punish Invidior for its greedy practices.
Suboxone’s original patent on its sublingual tablet was scheduled to expire in October 2009, which would have permitted consumers to buy cheaper, generic versions. However, Indivior’s parent company instructed them to create a new sublingual film version with identical ingredients to secure a fresh patent and remain the only provider of this type of opioid dependence drug.
Once the FDA approved the sublingual film version of Suboxone, Indivior’s parent company campaigned to pull the pill version from the market because it supposedly threatened child safety. In reality, Indivior was protecting its bottom line.4
We expect the final settlements to range from $50,000 to $150,000 or higher depending on your specific injuries.
Good proof of Suboxone’s adverse effects are dental x-rays from before and after starting the drug.
6. What are the side effects Suboxone?
The FDA website warns of the following side effects for Suboxone’s sublingual film:
- oral hypoesthesia (loss of mouth sensation),
- glossodynia (tongue pain),
- oral mucosal erythema (lesions),
- headache,
- nausea,
- vomiting,
- hyperhidrosis (excessive perspiration),
- constipation,
- signs and symptoms of withdrawal,
- insomnia,
- pain, and/or
- peripheral edema (swelling)
Dental problems are still not specifically listed.
7. Who makes Suboxone?
Suboxone is manufactured by the pharmaceutical company Indivior, Inc.
Indivior first made Suboxone in tablet form in 2002, which is when the FDA granted it “orphan drug” approval. This meant Indivior had exclusive marketing rights for several years.
Then on October 20, 2008, Indivior sought approval to change Suboxone’s form from pill to film. Indivior cited safety and efficiency studies, but really they were trying to prolong its lucrative patent protections.
The FDA finally approved Suboxone in film form on August 30, 2010. Then on July 2, 2013, the United States Patent and Trademark Office granted Indivior the patent for the film-based delivery of Suboxone.5
About Indivisor
Founded in 1994, Indivior has its headquarters in Richmond, Virginia. Indivior was originally a part of Reckitt Benckiser Pharmaceuticals, Inc. but separated in 2014.
Currently Indivior sells only three drugs in addition to Suboxone:
- Subutex, which also treats opioid addiction;
- Sublocade, which also treats opioid addiction, and
- Perseris (risperiodone), which treats schizophrenia.
In 2022, Indivior earned $901 million in revenue.6
Indivior anti-trust lawsuits
Recently Indivior settled several lawsuits for allegedly violating anti-trust laws by suppressing generic competition for Suboxone.
- On October 23, 2023 Indivior agreed to pay $385 million to settle lawsuits brought by drug wholesalers.7
- On August 21, 2023, Indivior agreed to pay $30 million to settle a class action lawsuit filed by health plans.8
- On July 24, 2020, Indivior agreed to pay $10 million to settle FTC (Federal Trade Commission) charges. (This $10 million is in addition to the $50 million settlement the FTC made with its prior parent company Reckitt Benckiser.) The FTC disbursed payments to about 54,448 class action members who had overpaid for Suboxone in the past.9
In July of 2019, Indivior pleaded guilty to felony criminal charges of deceptive marketing and agreed to pay $600 million. The company lied to physicians and health plans about Suboxone being safer and less prone to abuse than other addiction-suppressing drugs. Specifically, Indivior admitted that:
“In October 2012, it sought to convince MassHealth to expand Medicaid coverage of Suboxone Film in Massachusetts and sent MassHealth false data indicating that Suboxone Film had the lowest rate of accidental pediatric exposure (i.e., children taking medication by accident) of all buprenorphine drugs in Massachusetts, when in fact, it did not.”
Meanwhile, Indivior’s former parent company Reckitt Benckiser agreed to pay $1.4 billion to resolve its criminal and civil liability.10 Then two months later in October of 2019, Reckitt Benckiser agreed to pay $700 million to six states who sued them for improper marketing.11
8. What if I need Suboxone?
If you are currently a Suboxone user, contact your doctor. There may be alternative medications you can take to treat opioid addiction such as:
- Methadone
- Naltrexone
- Vivitrol (injectable naltrexone)
You may also wish to see a dentist to check for any signs of decay or other dental problems. These medical records will serve as valuable evidence if you end up filing a lawsuit.
If you have not yet taken Suboxone but decide with your doctor that it is the best medication for you, ask your dentist to:
- give you a baseline dental exam and a caries risk assessment;
- discuss with you how to prevent cavities (such as by brushing with fluoride toothpaste, flossing, using mouthwash, avoiding sugary foods, and consuming foods with calcium and vitamin D); and
- schedule you for regular checkups.
9. How does Suboxone work?
As a MOUD (medication for opioid use disorder), Suboxone prevents cravings by binding to your brain receptors.
Suboxone belongs to the class “narcotic analgesic combinations,” and its key ingredients are:
- buprenorphine, which is taken as a replacement in the treatment of drug dependence, and
- naloxone, which reverses opioid overdoses.12
The purpose of Suboxone is to help opioid addicts
- beat their addiction,
- reduce their risk of overdosing, and
- return to a normal life.
10. How can an attorney help me?
Our Suboxone lawyers take care of the whole legal process so you can focus on getting treatment for your dental issues and moving on with your life. Specifically, we:
- Compile all your medical/dental records and any other relevant evidence.
- Research and write all the necessary court filings and legal correspondence throughout the negotiation and litigation process. There is a good possibility that you will never have to sit for a deposition or appear in court.
- Hold strategic settlement talks with Indivior in pursuit of the biggest financial settlement possible.
Indivior’s defense attorneys can seem daunting. Plus they bill hourly, so they are in no rush to settle Suboxone lawsuits.
However, we know how to hold their feet to the flames and expedite the court process so that you walk away with the maximum payout as soon as possible. Since we do not get paid unless we win, you have nothing to lose and everything to gain.

Speak with your doctor about safer alternatives to treat opioid use disorder.
Legal References
- Etminan et al., Association Between Sublingual Buprenorphine-Naloxone Exposure and Dental Disease, JAMA (2022) (a retrospective cohort study). See also: Two prospective survey studies discovered that the health service most requested by patients in buprenorphine treatment programs were dental services. (Winstock et al., Patients’ help-seeking behaviours for health problems associated with methadone and buprenorphine treatment, Drug. Alcohol Rev (July, 2008); Abbott, Case management: ongoing evaluation of patients’ needs in an opioid treatment program, Prof Case Manag. (May – June, 2010)). Three disproportionality analyses showed a high frequency of reporting of dental disorders to FAERS (Woods, Dental Disorders Reported to the FDA Adverse Event Reporting System in Association with Buprenorphine: An Analysis by Ingredient Composition and Route of Administration, Curr Drug Saf (July 31, 2023)) and VigiBase (de Campaigno et al., Drug-Induced Dental Caries: A Disproportionality Analysis Using Data from VigiBase, Drug Safety (July, 2017); Barus et al., Sublingual/Buccal buprenorphine and dental problems: a pharmacovigilance study (August, 2023)) by buprenorphine patients. One case series (Suzuki et al., Sublingual buprenorphine and dental problems: a case series, Prime Care Companion CNS Discord (2013)) and one case report (Suzuki & Park, Buprenorphine/naloxone and dental caries: a case report, Am J Addict (2012)) documented dental problems in people taking buprenorphine or buprenorphine/naloxone.
- Melissa Busch, Man sues opioid use disorder drug maker for causing tooth decay, drbicuspid.com (October 2, 2023).
- See same.
- See Tracy Staton, Reckitt to pull Suboxone tabs in favor of on-patent film version, Fierce Pharma (September 25, 2012).
- See notes 2 and 4. Reckitt Benckiser Pharms., Inc. v. Biodelivery Scis. Int’l, Inc. (E.D.N.C. May 20, 2014) No. 5:13-CV-760-BO.
- Reckitt Benkiser says Indivior split off to complete Dec 23, Reuters (November 16, 2014). FY 2022 Financial Results Announced – Executed Against Our Growth Strategy, Indivior (February 16, 2023).
- Indivior to pay $385 million to end final Suboxone monopoly lawsuits, Reuters (October 24, 2023)
- Indivior to pay $30 million to settle health plans’ Suboxone claims, Reuters (October 21, 2023).
- Indivior, Inc. to Pay $10 Million to Consumers, Settling FTC Charges that the Company Illegally Maintained a Monopoly over the Opioid Addiction Treatment Suboxone, FTC (July 24, 2020).
- Indivior Solutions Pleads Guilty To Felony Charge And Indivior Entities Agree To Pay $600 Million To Resolve Criminal And Civil Investigations As Part Of DOJ’s Largest Opioid Resolution, U.S. Department of Labor (July 24, 2020).
- Erik Larson, Drug Distributor Reckitt to Pay $700 Million Over Opioid Marketing, Bloomberg (October 23, 2019).
- See Medications for Opioid Use Disorder Study (MOUD Study), Centers for Disease Control (CDC). Narcotic analgesic combinations, Drugs.com (April 14, 2023).
