Neurovascular stent lawsuits are mass tort and medical malpractice claims against doctors, surgeons, and the makers of neurovascular stents for their negligence and their failure to warn people of the risks of using these stents to treat brain aneurysms. The medical procedures that insert these stents into an artery in the brain are risky endeavors, especially for certain people at a heightened risk of suffering a stroke during or after the procedure. Nevertheless, doctors have recommended neurovascular stents to at-risk patients. Victims who have been hurt by these stents have filed lawsuits, seeking compensation for their:
- Medical expenses,
- Lost wages and other professional setbacks, and
- Pain and suffering.
- 1. What is a neurovascular stent?
- 2. The dangers of neurovascular stents
- 3. Lawsuits over injuries from neurovascular stents
- 4. Compensation available to victims of neurovascular stent injuries
1. What is a neurovascular stent?
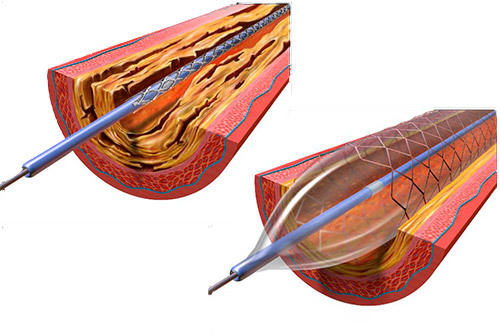
A neurovascular stent is a medical device that is used to treat brain aneurysms without the need for invasive surgery.
Contrary to popular belief, a brain aneurysm is not a burst blood vessel in the brain. Instead, an aneurysm is just a weakened wall of an artery. Because the wall of the artery is weak and compromised, the artery bulges outward from the pressure of the blood flow inside the artery. These bulging arteries carry a risk of rupturing – becoming the burst blood vessel that many people think is an aneurysm.
Not all aneurysms are the same, though. Some bulging arteries are “tall” but “narrow” – they bulge far outwards from the artery, but only a tiny section of the artery is affected. Others are “short” but “wide” – there is only a small bulge, but a considerable section of the artery is affected, sometimes several millimeters in length. These wide aneurysms carry a higher risk of rupturing, making them far more severe medical conditions.
When aneurysms happen in the brain, they can be fatal if they burst: The sudden flow of blood into the tissues of the brain can quickly cause irreparable damage.
Neurovascular stents can be used to treat aneurysms and prevent them from rupturing. They are especially useful when the aneurysm is in the brain.
A neurovascular stent is a tiny coil of metal or plastic – much like a spring – that gets inserted into the artery and navigated to the aneurysm’s location. Once there, the coil is expanded outwards in the artery, where it provides support for the weakened arterial walls.1
Where at least four millimeters of the artery have bulged outwards in an aneurysm – a “wide-necked” bulge – the stent alone is unlikely to provide enough support. In these cases, doctors and surgeons can turn to the process of stent-assisted coiling. This process inserts the neurovascular stent into the artery at the location of the aneurysm and then fills the bulging section of the artery with another coil. This additional coil induces blood clotting in the aneurysm, effectively sealing it off and preventing blood from flowing into it. The stent keeps this coil in place.
When done properly and coupled with blood-thinning medication, neurovascular stents and stent-assisted coiling can provide the support the artery needs, preventing the aneurysm from bursting and causing serious damage.
2. The dangers of neurovascular stents
Neurovascular stent procedures, while they are less invasive than a traditional surgery to treat a brain aneurysm, are still intensive medical procedures that pose serious risks for people who are already susceptible to strokes, or to people who cannot take the blood-thinning medications that neurovascular stent procedures often require. They can also be improperly done in ways that lead to serious complications for patients.
Several studies have found that neurovascular stents can create serious complications for susceptible patients.2 Some of these studies drew attention to the risks as early as 1998. Complications arising from neurovascular stents included:
- Strokes, both during and immediately after the stent was implanted,
- Migration of the stent, leading to arterial damage or a rupture of the aneurysm, and
- Death.
However, was not until May 7, 2018 that the U.S. Food and Drug Administration (FDA) issued a letter to doctors and surgeons about the risks associated with neurovascular stent procedures.3
This letter warned health care providers about the increased risks of neurovascular stents for patients who had health problems that have shortened their life expectancy, as well as for patients who could not take blood-thinning drugs after the procedure. The letter recommended using alternative forms of treatment for smaller aneurysms.
The warning also advised doctors to more carefully select patients for neurovascular stent procedures based on the odds that the aneurysm could rupture. According to the FDA, only higher risk patients should be considered for a neurovascular stent procedure. Factors to be considered included:
- Patient’s age,
- Known aneurysm symptoms like cranial nerve deficit,
- Family or personal history for aneurysms,
- Gender,
- Ethnicity,
- History of smoking tobacco,
- Hypertension, and
- Location, size, and changes in the aneurysm.4
Lower risk patients whose aneurysms did not pose an immediate risk should be treated more conservatively, using medication and close monitoring for further developments.

3. Lawsuits over injuries from neurovascular stents
Since the FDA issued its letter to health care providers, lawsuits have been filed against doctors, surgeons, and stent manufacturers. These lawsuits claim that the victim suffered severe harm, through no fault of their own, because of someone else’s negligence. The lawsuits demand compensation for the victim’s injuries, or for losses suffered by the victim’s family.
The lawsuits that have been filed against doctors and surgeons claim that inserting the neurovascular stent was so poorly done that it amounted to medical malpractice. These lawsuits can make use of two different arguments:
- The surgeon failed to properly insert the neurovascular stent or the stent-assisted coil, leading to serious and life-threatening complications when the stent moved or failed, or
- The doctor should never have recommended a neurovascular stent or stent-assisted coil for the patient’s aneurysm, given the patient’s medical history or the severity of the aneurysm.
In either case, the negligence of the doctor or surgeon caused the patient’s injuries from the neurovascular stent. The lawsuit seeks to hold them accountable for their poor conduct.
Lawsuits have also been filed against the manufacturers of neurovascular stents, as well. These lawsuits claim that these companies did not uphold their legal duty to disclose the dangers of using their products.
Medical device companies have a legal obligation to tell doctors and patients of the risks of using their device. However, doing so alerts medical professionals to these dangers, which can hurt the sales of the device. This provides a financial incentive for medical device makers to not disclose the dangers of their products, and even to cover up the risks.
The lawsuits against the makers of neurovascular stents claim that this is just what happened. They seek compensation from the companies that design and sell the neurovascular stents that were used to treat their aneurysm, including:
- Stryker Neurovascular,
- Covidien and Medtronic,
- Integer, and
- Johnson & Johnson.
4. Compensation available to victims of neurovascular stent injuries
People who have been hurt by neurovascular stents deserve compensatory damages for their losses, including their:
- Medical expenses accrued during their recovery,
- Future medical expenses that are anticipated for their care,
- Lost wages,
- Reduced ability to earn a living because of their injuries,
- Physical pain,
- Mental suffering, and
- Their family’s loss of companionship.
Additionally, people who have lost a loved one to complications from a neurovascular stent procedure can file a wrongful death claim for compensation.
Legal References:
- See Novitzke J, “A Patient Guide to Brain Stent Placement,” Journal of Vascular and Interventional Neurology 2(2):177-9 (April 2009).
- See Rinkel GJ, Djibuti M, Algra A, van Gijn J, “Prevalence and risk of rupture of intracranial aneurysms: a systematic review,” Stroke 29(1):251-6 (January 1998); Wiebers D, “Unruptured Intracranial Aneurysms — Risk of Rupture and Risks of Surgical Intervention,” The New England Journal of Medicine 339:1725-33 (December 10, 1998); Thompson G et al., “Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms,” Stroke 46(8):2368-2400 (June 18, 2015).
- FDA, “Letter to Health Care Providers: Neurovascular Stents Used for Stent-Assisted Coiling of Unruptured Brain Aneurysms,” (May 7, 2018).
- See note 3.
